Science’s COVID-19 reporting is supported by the Pulitzer Center and the Heising-Simons Foundation.
Athena Akrami’s neuroscience lab reopened last month without her. Life for the 38-year-old is a pale shadow of what it was before 17 March, the day she first experienced symptoms of the novel coronavirus. At University College London (UCL), Akrami’s students probe how the brain organizes memories to support learning, but at home, she struggles to think clearly and battles joint and muscle pain. “I used to go to the gym three times a week,” Akrami says. Now, “My physical activity is bed to couch, maybe couch to kitchen.”
Her early symptoms were textbook for COVID-19: a fever and cough, followed by shortness of breath, chest pain, and extreme fatigue. For weeks, she struggled to heal at home. But rather than ebb with time, Akrami’s symptoms waxed and waned without ever going away. She’s had just 3 weeks since March when her body temperature was normal.
“Everybody talks about a binary situation, you either get it mild and recover quickly, or you get really sick and wind up in the ICU,” says Akrami, who falls into neither category. Thousands echo her story in online COVID-19 support groups. Outpatient clinics for survivors are springing up, and some are already overburdened. Akrami has been waiting more than 4 weeks to be seen at one of them, despite a referral from her general practitioner.
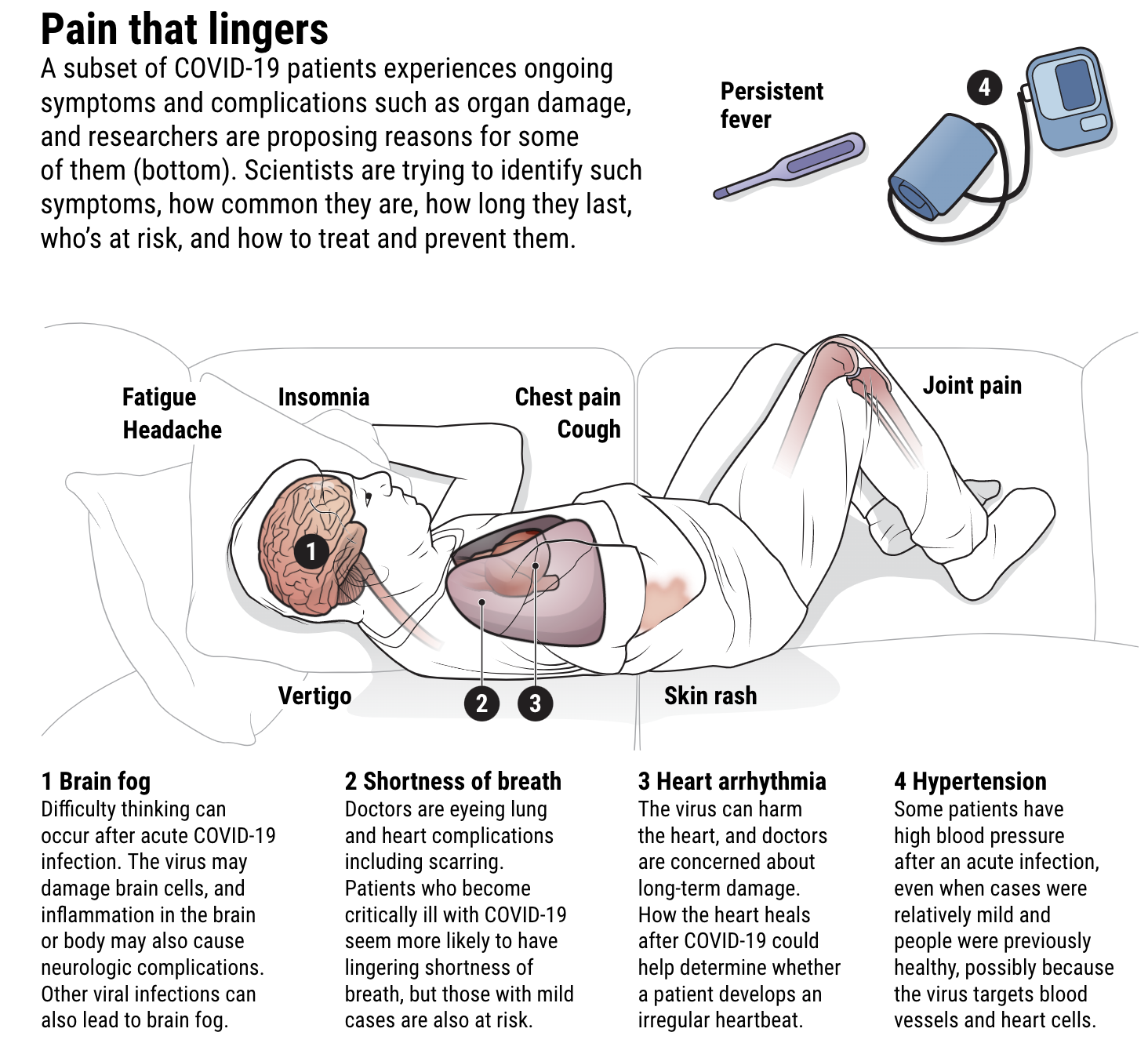
The list of lingering maladies from COVID-19 is longer and more varied than most doctors could have imagined. Ongoing problems include fatigue, a racing heartbeat, shortness of breath, achy joints, foggy thinking, a persistent loss of sense of smell, and damage to the heart, lungs, kidneys, and brain.
The likelihood of a patient developing persistent symptoms is hard to pin down because different studies track different outcomes and follow survivors for different lengths of time. One group in Italy found that 87% of a patient cohort hospitalized for acute COVID-19 was still struggling 2 months later. Data from the COVID Symptom Study, which uses an app into which millions of people in the United States, United Kingdom, and Sweden have tapped their symptoms, suggest 10% to 15% of people—including some “mild” cases—don’t quickly recover. But with the crisis just months old, no one knows how far into the future symptoms will endure, and whether COVID-19 will prompt the onset of chronic diseases.
Researchers are now facing a familiar COVID-19 narrative: trying to make sense of a mystifying illness. Distinct features of the virus, including its propensity to cause widespread inflammation and blood clotting, could play a role in the assortment of concerns now surfacing. “We’re seeing a really complex group of ongoing symptoms,” says Rachael Evans, a pulmonologist at the University of Leicester.
Survivor studies are starting to probe them. This month, researchers across the United Kingdom including Evans launched a study that will follow 10,000 survivors for 1 year to start, and up to 25 years. Ultimately, researchers hope not just to understand the disease’s long shadow, but also to predict who’s at highest risk of lingering symptoms and learn whether treatments in the acute phase of illness can head them off.
For Götz Martin Richter, a radiologist at the Klinikum Stuttgart in Germany, what’s especially striking is that just as the illness’ acute symptoms vary unpredictably, so, too, do those that linger. Richter thinks of two patients he has treated: a middle-aged man who experienced mild pneumonia from COVID-19, and an elderly woman already suffering from chronic leukemia and arterial disease, who almost died from the virus and had to be resuscitated. Three months later, the man with the mild case “falls asleep all day long and cannot work,” Richter says. The woman has minimal lung damage and feels fine.
EARLY IN the pandemic, doctors learned that SARS-CoV-2, the virus that causes COVID-19, can disrupt a breathtaking array of tissues in the body. Like a key fitting neatly into a lock, SARS-CoV-2 uses a spike protein on its surface to latch onto cells’ ACE2 receptors. The lungs, heart, gut, kidneys, blood vessels, and nervous system, among other tissues, carry ACE2 on their cells’ surfaces—and thus, are vulnerable to COVID-19. The virus can also induce a dramatic inflammatory reaction, including in the brain. Often, “The danger comes when the body responds out of proportion to the infection,” says Adrija Hajra, a physician at Albert Einstein College of Medicine in New York City. She continues to care for those who were infected in the spring and are still recovering.
Despite the novelty of SARS-CoV-2, its long-term effects have precedents: Infections with other pathogens are associated with lasting impacts ranging from heart problems to chronic fatigue. “Medicine has been used to dealing with this problem” of acute viral illness followed by ongoing symptoms, says Michael Zandi, a neurologist at UCL. Even common illnesses such as pneumonia can mean a monthslong recovery. “I see a lot of people who had [the brain inflammation] encephalitis 3, 4 years ago, and still can’t think, or are tired,” Zandi says. Infections with certain bacteria and Zika virus, among others, are linked to Guillain-Barre syndrome, in which the immune system attacks nerve tissue, causing tingling, weakness, and paralysis. (Some cases of Guillain-Barre after COVID-19 have been reported, but “it’s not definite [there’s] a spike,” says Rachel Brown, a UCL neurologist who works with Zandi.)
Based on experience with other viruses, doctors can “extrapolate and anticipate” potential long-term effects of COVID-19, says Jeffrey Goldberger, chief of cardiology at the University of Miami. Like SARS-CoV-2, some other viruses, such as Epstein-Barr, can damage heart tissue, for example. In those infections, the organ sometimes heals completely. Sometimes, scarring is mild. “Or,” Goldberger says, “it could be severe and lead to heart failure.”
Michael Marks, an infectious disease specialist at the London School of Hygiene & Tropical Medicine who’s helping lead the U.K. survivor study, says he’s not too surprised at emerging aftereffects. “What we’re experiencing is an epidemic of severe illness,” he says. “So therefore, there is an epidemic” of chronic illness that follows it.
But outcomes following SARS-CoV-2 also appear distinct in ways both hopeful and dispiriting. Early this year, many doctors feared the virus would induce extensive, permanent lung damage in many survivors because two other coronaviruses, the viruses that cause the first severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome, can devastate the lungs. One study of health care workers with SARS in 2003 found that those with lung lesions 1 year after infection still had them after 15 years.
“We expected to see a lot of long-term damage from COVID-19: scarring, decreased lung function, decreased exercise capacity,” says Ali Gholamrezanezhad, a radiologist at the Keck School of Medicine at the University of Southern California who in mid-January began to review lung scans from COVID-19 patients in Asia. Hundreds of scans later, he has concluded that COVID-19 ravages the lungs less consistently and aggressively than SARS did, when about 20% of patients sustained lasting lung damage. “COVID-19 is in general a milder disease,” he says.
At the same time, the sheer breadth of complications linked to COVID-19 is mind-boggling. In late April, Akrami collaborated with Body Politic, a group of COVID-19 survivors, to survey more than 600 who still had symptoms after 2 weeks. She logged 62 different symptoms and is now readying the findings for publication and developing a second survey to capture longer term ailments. “Even though it’s one virus, it can cause all different kinds of diseases in people,” says Akiko Iwasaki, an immunologist at Yale University who is studying lingering effects on the immune system.
BY NOW IT’S CLEAR that many people with COVID-19 severe enough to put them in a hospital face a long recovery. The virus ravages the heart, for example, in multiple ways. Direct invasion of heart cells can damage or destroy them. Massive inflammation can affect cardiac function. The virus can blunt the function of ACE2 receptors, which normally help protect heart cells and degrade angiotensin II, a hormone that increases blood pressure. Stress on the body from fighting the virus can prompt release of adrenaline and epinephrine, which can also “have a deleterious effect on the heart,” says Raul Mitrani, a cardiac electrophysiologist at the University of Miami who collaborates with Goldberger.
Mitrani and Goldberger, who co-authored a June paper in Heart Rhythm urging follow-up of patients who might have heart damage, worry in particular about the blood enzyme troponin, which is elevated in 20% to 30% of hospitalized COVID-19 patients and signifies cardiac damage. (Troponin is sky-high during a heart attack, for example.) How the heart heals following COVID-19 might determine whether an irregular heartbeat develops or persists, Goldberger believes. “We have one guy in the hospital right now who had COVID 2 months ago and had all sorts of arrhythmia problems” then, Goldberger says. “He’s recovered from his COVID, but still has the arrhythmia.” For some patients with coronavirus-induced heart problems, treatments as simple as cholesterol-lowering drugs, aspirin, or beta blockers could help, Goldberger says.
Many people the pair has seen with heart complications post–COVID-19 had preexisting conditions, most commonly diabetes and hypertension. COVID-19, Goldberger suspects, tips them into more hazardous terrain or accelerates the onset of heart problems that, absent the coronavirus, might have developed later.
But other patients are affected without apparent risk factors: A paper this week in JAMA Cardiology found that 78 of 100 people diagnosed with COVID-19 had cardiac abnormalities when their heart was imaged on average 10 weeks later, most often inflammation in heart muscle. Many of the participants in that study were previously healthy, and some even caught the virus while on ski trips, according to the authors.
Severe lung scarring appears less common than feared—Gholamrezanezhad knows of only one recovered patient who still needs oxygen at rest. Scarring seems most likely to accompany underlying lung disease, hypertension, obesity, and other conditions. Lung damage is also seen in people who spend weeks on a ventilator. Gholamrezanezhad suspects that, as with harm to the heart, previously healthy people are not exempt from the virus’ long-term effects on the lungs, though their risk is likely lower.
Then there’s the nervous system, a worrying target. Severe complications seem relatively rare but aren’t limited to those whom the virus renders critically ill. Brown, Zandi, and colleagues described 43 people with neurologic complications this month in Brain; many had been hospitalized during their acute infection, but not always for long—and for some, neurologic problems were their most debilitating symptom and the reason for hospital admission. Several were struggling to recover from encephalitis. Others had inflammation in their brain’s white matter, which helps transmit electrical signals.
Separately, doctors are starting to see a class of patients who, like Akrami, struggle to think clearly—another outcome physicians have come upon in the past. After some severe viral infections, there are “those people who still don’t feel quite right afterward, but have normal brain scans,” Brown says. Some neurologists and patients describe the phenomenon as “brain fog.” It’s largely a mystery, though one theory suggests it’s similar to a “postviral fatigue related to inflammation in the body,” Brown says.
Could that be happening here? “Who knows, really?” Brown asks. “These patients need to be followed.”
PEOPLE LIKE THESE pose a growing concern (though they are also often dismissed by physicians). Collectively, these “long-haulers” describe dozens of symptoms, including many that could have multiple causes, such as fatigue, joint pain, and fever. “It’s time to give some voice to this huge population of patients,” Akrami says.
The most bedeviling and common lingering symptom seems to be fatigue, but researchers caution against calling it chronic fatigue syndrome. That’s “a specific diagnosis,” Marks says. “You might have fibrosis in the lungs, and that will make you feel fatigued; you might have impaired heart function, and that will make you feel fatigued.” Trying to trace symptoms to their source is critical to understanding and ultimately managing them, he says.
Iwasaki agrees. Doctors would treat symptoms differently depending on whether they result from a lingering infection or are rooted in autoimmune abnormalities. She has begun to recruit people who weren’t hospitalized when they had COVID-19 and will sift through her volunteers’ immune cells, examine whether they’re primed to attack, and measure whether the balance among different cell types is as it should be. She’ll also hunt for virus in saliva. “We’re pretty much searching for anything,” she says.
Iwasaki is especially struck by the number of young, healthy, active people—people like Akrami—who fall into the long-hauler category. As she and others struggle to find ways to help them, she wonders what might head off their symptoms. One possibility, she says, is monoclonal antibodies, which are now being tested as a treatment for acute infection and might also forestall lasting immune problems.
Hers is one of several survivor studies now underway. While Goldberger’s hometown of Miami faces a surge of acutely ill patients, he is looking ahead, applying for funding to image the heart and map its electrical activity in COVID-19 patients after they leave the hospital. Gholamrezanezhad is recruiting 100 patients after hospital discharge to follow for up to 2 years for lung assessments. Like many physicians, he fears the societal impact of even uncommon complications, including in the millions of people never hospitalized. “When you consider how many people are getting the disease, it’s a big problem,” he says.
Across the Atlantic Ocean, Richter has recruited 300 volunteers in Germany for long-term follow-up, including lung scans. In the United Kingdom, patients will soon be able to sign up for that country’s survivor study, with many giving blood samples and being examined by specialists. The researchers will probe patients’ DNA and examine other characteristics such as age and health history to learn what might protect them from, or make them susceptible to, a range of COVID-19 induced health problems. Knowing who’s at risk of, say, kidney failure or cardiac arrhythmia could mean more targeted follow-up. The U.K. researchers are also keen to see whether patients who received certain treatments in the acute phase of illness, such as steroids or blood thinners, are less prone to later complications.
For her part, Akrami is one of 2 million people infected weeks or months ago participating in the COVID Symptom Study. The study welcomes anyone infected, and with 10% to 15% of people who use the app reporting ongoing symptoms, it has already yielded a welter of data, says Andrew Chan, an epidemiologist and physician at Harvard Medical School.
As he and his colleagues parse the data, they are identifying distinct “types” of acute illness, based on clusters of symptoms. Chan wonders whether certain early symptoms correlate with specific ones that linger. He acknowledges the risk that the app’s data could be skewed, because people who aren’t feeling well may be more likely to participate than those who have smooth recoveries. “We’re trying to develop data analysis tools” to account for that tilt, he says, “similar to methods used in polling. You have to weigh the biases.”
One of the few systematic, long-term studies of COVID-19 patients with mild acute symptoms is underway in San Francisco, where researchers are recruiting 300 adults from local doctors and hospitals, for 2 years of follow-up. “We don’t have a broad idea of what’s happening” after the initial illness, says Steven Deeks, an HIV researcher at the University of California, San Francisco, who is leading the study, modeled on HIV cohorts he has followed for decades. What does “ongoing symptoms” even mean, Deeks asks. “Is that weeks, months? We don’t know that it’s years.”
More than 100 people ranging in age from 18 to 80 have signed up so far. Cardiologists, neurologists, pulmonologists, and others are assessing the volunteers, and blood, saliva, and other biological specimens are being banked and analyzed.
Although scientists hope they’ll learn how to avert chronic symptoms and help patients currently suffering, this latest chapter in the COVID-19 chronicle has been sobering. The message many researchers want to impart: Don’t underestimate the force of this virus. “Even if the story comes out a little scary, we need a bit of that right now,” Iwasaki says, because the world needs to know how high the stakes are. “Once the disease is established, it’s really hard to go backward.”
COVID-19 Update: The connection between local and global issues–the Pulitzer Center's long standing mantra–has, sadly, never been more evident. We are uniquely positioned to serve the journalists, news media organizations, schools and universities we partner with by continuing to advance our core mission: enabling great journalism and education about underreported and systemic issues that resonate now–and continue to have relevance in times ahead. We believe that this is a moment for decisive action. Learn more about the steps we are taking.