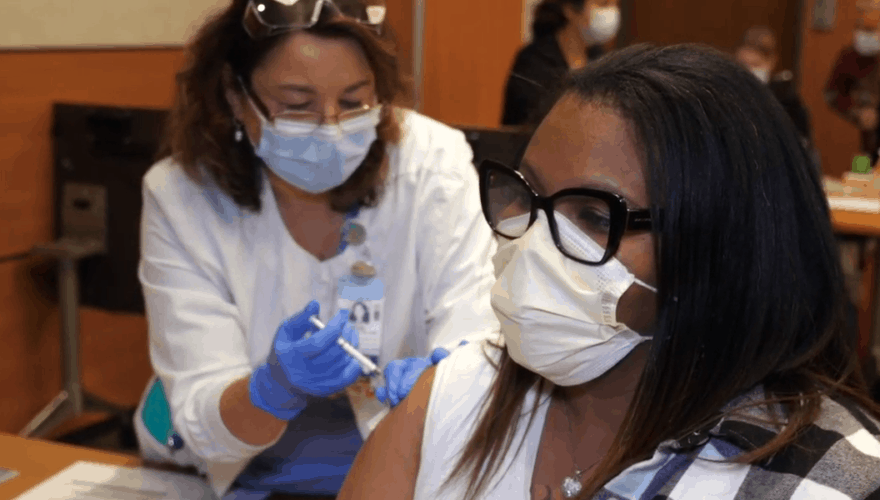
When I first became interested in taking part in a coronavirus vaccine trial, I assumed all I had to do was step forward. I believed that the same characteristics that raised my coronavirus risk — Black, over 60, type 2 diabetic — also made me highly sought-after as a volunteer. But it wasn’t nearly that easy.
North Carolina was one of the first states to release coronavirus data by race. The numbers from Charlotte/Mecklenburg County, where I live, showed the disparate impact of the virus on people of color and were soon confirmed by the skyrocketing COVID-19 rates in other cities with high Black populations.
Then the myth began circulating on social media that Black people were somehow immune to the disease and five people sent me a link to the Plandemic conspiracy-theory video in one day. I could see how the Black community’s well-justified skepticism about public health initiatives would exacerbate the health crisis we were facing.
In mid-summer, vaccine manufacturers Pfizer and Moderna announced they would recruit 60,000 participants — 18 and older, all genders and racial and ethnic groups — for their late-stage clinical trials. Volunteering was a way to ensure that Blacks were adequately represented in the research and demonstrate that the vaccine is safe for this community.
The ethical dilemma
While some people thanked me for being willing to risk my personal safety to advance medical science, others expressed surprise and even outrage. How could I offer myself up as a human guinea pig to the very medical establishment that alternates between ignoring and exploiting Black people?
Without guinea pigs, where would we be, I asked rhetorically in response.
A Washington Post poll conducted in the spring found that more than 30 percent of Blacks personally know someone who died from COVID-19. Yet, a December Pew study found that only 42 percent of Black adults said they would be willing to receive the vaccine, down two points from a survey taken in mid-June.
Why diversity matters
Black people have many reasons to distrust a public health system that has used our bodies for experimentation without care or consent. But given the coronavirus’s impact on the Black community, informed participation in clinical trials is essential to ensure the vaccine works, regardless of age, gender and ethnic background.
Little has changed since 1993, when Congress passed the NIH Revitalization Act, which requires federally funded research projects to include women and minorities in their trials. In fact, the numbers appear to be headed in the wrong direction.
People of color account for fewer than 10 percent of patients enrolled in clinical trials, according to the National Institute on Minority Health and Health Disparities. There has been a longstanding assumption that the problem lies in minorities’ distrust of the medical establishment because of historic mistreatments during the Tuskegee study and the eugenics movement. While lack of trust is a factor, a recent study published in the American Cancer Society Journals found bias among health care professionals as a contributing factor.
Some study recruiters perceived race as irrelevant when screening and recruiting potential minority participants. Others not only viewed racial and ethnic minorities as less promising participants, some reported withholding trial opportunities from minorities based on these perceptions.
Pfizer and Moderna touted diversity as a priority for their phase 3 coronavirus vaccine trials. I responded to several solicitations looking for volunteers in the Charlotte area, feeling confident I would be picked.
If selected, I would receive two shots, an initial vaccine (or placebo), then a booster shot (or placebo) about a month later. I would be required to document any side effects from the vaccination in an electronic diary.
Those side effects commonly include pain or soreness near the injection site, headaches, muscle aches, fatigue and fevers.
Who gets chosen?
My white friends Anita and Lucia were both selected for the Pfizer trial. Retired manufacturing executive Lucia was recruited because she was in their database after participating in a study for people with celiac disease a year earlier. Anita, a psychology professor, told me she wanted to put her “body on the line to help others.”
Anita believes she got the vaccine and not a placebo because she developed a mild fever (99.5 to 100.0) the day after her first injection. “I usually don’t run fevers and haven’t had once since,” she says. Lucia thinks she got the placebo because she had no side effects after her shot.
As I stood by for the call that never came, in early October, Moderna announced that it had failed to recruit enough Black, Latino and Native American participants in its study. To make up for the shortfall, the firm slowed enrollment and instructed research centers to increase participation among minority volunteers.
Frustrated, I applied again (clearly identifying myself as Black this time) and waited. Still nothing.
I wasn’t willing to give up but clearly, this was going to be much harder than I imagined.
COVID-19 Update: The connection between local and global issues–the Pulitzer Center's long standing mantra–has, sadly, never been more evident. We are uniquely positioned to serve the journalists, news media organizations, schools, and universities we partner with by continuing to advance our core mission: enabling great journalism and education about underreported and systemic issues that resonate now–and continue to have relevance in times ahead. We believe that this is a moment for decisive action. Learn more about the steps we are taking.