Science’s COVID-19 reporting is supported by the Pulitzer Center and the Heising-Simons Foundation.
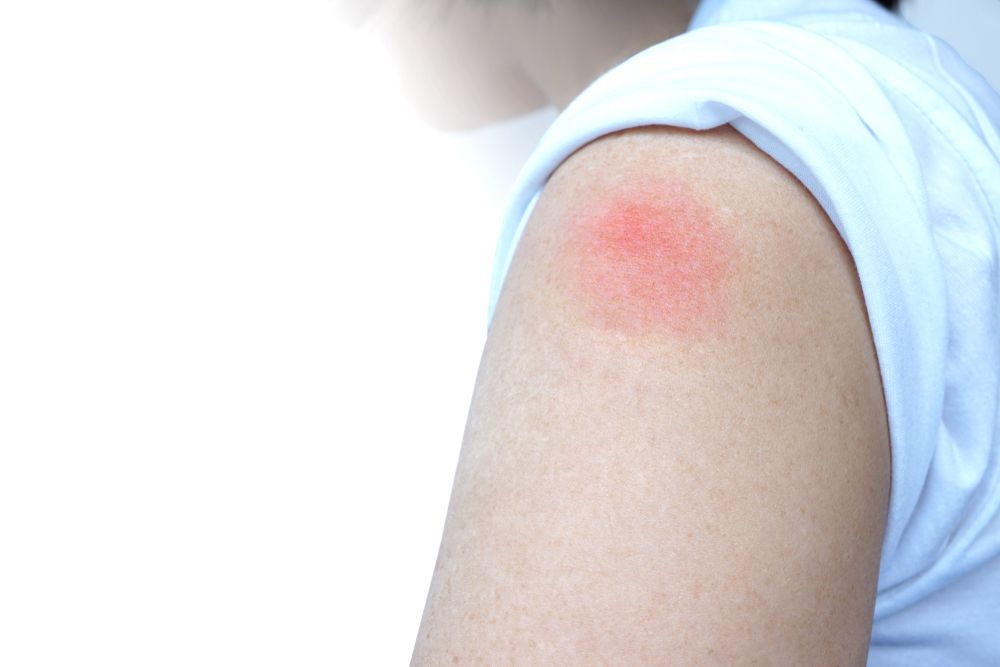
This summer, Luke Hutchison, a Massachusetts Institute of Technology–educated computational biologist, volunteered for a trial of Moderna’s COVID-19 vaccine. After he got the second injection, his arm immediately swelled up to the size of a “goose egg,” Hutchison says. He can’t be sure he got the vaccine and not a placebo, but within a few hours, the healthy then-43-year-old was beset by bone and muscle aches and a 38.9°C fever that felt, he says, “unbearable.” “I started shaking. I had cold and hot rushes,” he says. “I was sitting by the phone all night long thinking: ‘Should I call 911?’”
Hutchison’s symptoms resolved after 12 hours. But, he says, “Nobody prepared me for the severity of this.”
He says the public should be better prepared than he was, because a subset of people may face intense, if transient, side effects, called reactogenicity, from Moderna’s vaccine. Some health experts agree.
“Somebody needs to address the elephant: What about vaccine reactogenicity? While it’s safe, [and] it’s not going to cause any long-term issues … how is that perception going to go with the public once they start receiving it?” says Deborah Fuller, a vaccinologist at the University of Washington, Seattle, whose lab is developing second-generation RNA vaccines against COVID-19. She worries the side effects could feed vaccine hesitancy. “I feel like it’s being glossed over.”
Those concerns arise after a week of good news about coronavirus vaccines: Both Moderna and Pfizer, with BioNTech, announced that their messenger RNA (mRNA) vaccines reached 95% efficacy in clinical trials of tens of thousands of people. The trials revealed no serious safety concerns, both companies added.
Both vaccines consist of a snippet of genetic code directing production of the coronavirus’ spike protein, delivered in a tiny fat bubble called a lipid nanoparticle. Some suspect the immune system’s response to that delivery vehicle is causing the short-term side effects.
Those transient reactions should not dissuade people from getting vaccinated in the face of a pandemic virus that kills at least one in 200 of those it infects, says Florian Krammer, a vaccinologist at the Icahn School of Medicine at Mount Sinai, who participated in Pfizer’s pivotal trial. Sore arms, fevers, and fatigue are “unpleasant but not dangerous. I’m not concerned about [reactogenicity],” he says.
And most people will escape “severe” side effects, defined as those that interfere with daily activity. Fewer than 2% of recipients of the Pfizer and Moderna vaccines developed severe fevers of 39°C to 40°C. But if the companies win U.S. Food and Drug Administration approvals, they’re aiming to supply vaccine to 35 million people in the United States by the end of December. If 2% experienced severe fever, that would be 700,000 people.
Other transient side effects would likely affect even more people. The independent board that conducted the interim analysis of Moderna’s huge trial found that severe side effects included fatigue in 9.7% of participants, muscle pain in 8.9%, joint pain in 5.2%, and headache in 4.5%. For the Pfizer/BioNTech vaccine, the numbers were lower: Severe side effects included fatigue (3.8%) and headache (2%).
That’s a higher rate of severe reactions than people may be accustomed to. “This is higher reactogenicity than is ordinarily seen with most flu vaccines, even the high-dose ones,” says Arnold Monto, an epidemiologist at the University of Michigan School of Public Health.
Front-line public health workers should prepare their messages, says Bernice Hausman, an expert on vaccine controversy at the Pennsylvania State University College of Medicine. “Public health professionals are going to have to have a story that gets out in front of [stories like Hutchison’s]—that responds to the way that people are going to try to make that a story about vaccine injury.”
Transparency is key, Hausman emphasizes. Rather than minimizing the chance of fever, for instance, vaccine administrators could alert people that they may experience a fever that can feel severe but is temporary. “That would go a significant way toward people feeling like they are being told the truth.” Adds Drew Weissman, an immunologist at the University of Pennsylvania whose work contributed to both vaccines: “The companies just have to warn people: ‘This is what you need to expect. Take Tylenol and suck it up for a day.’”
Hausman also sees a need to support people who have serious reactions. “The real question is whether or not there is going to be an apparatus set up to support the experience of people going through [experiences like Hutchison’s]. Like a hotline with a nurse triaging … and figuring out if you need to go to the hospital or not. Will your medical expenses be covered if you do? These are important questions.”
Both Moderna’s and Pfizer/BioNTech’s vaccines require two doses separated by several weeks. Reactogenicity is typically higher after a second dose, Weissman says. The side effects “mean the vaccine is working well. … [It] means you had such a good immune response to the first dose and now you are seeing the effects of that,” he says. (Weissman co-invented the mRNA modifications that both Moderna and BioNTech have licensed to make their vaccines, and he receives royalties from the companies.)
“We suspect the lipid nanoparticle causes the reactogenicity, because lipid nanoparticles without mRNA in them do the same thing in animals,” Weissman says. “We see production, in the muscle, of inflammatory mediators that cause pain, [redness], swelling, fever, flulike symptoms, etc.”
Ian Haydon, who received the highest dose of the Moderna vaccine in its first human trial, knows what that’s like. (He received 250 micrograms, but partly in response to reactions like his, the company chose to take forward a lower dose of 100 micrograms.)
Twelve hours after receiving his second injection in May, Haydon got chills as well as “headache, muscle ache, fatigue, nausea,” and had a fever of 39.6°C. He went to urgent care, and later vomited and fainted before the symptoms receded, roughly 24 hours after they started, he says.
But Haydon says his experience was “a small price to pay” for the possibility of returning to normal life. “For me, this was a rough day. But if you compare it to what COVID can do, I think it really pales in comparison.”
Longer term side effects of mRNA vaccines remain theoretical. They include the possibility that people with lupus, whose disease is driven by antibodies against their own genetic code, could experience flare-ups because of the revved up immune response induced by the vaccines, says Sarfaraz Hasni, director of lupus clinical research at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
But there’s no evidence currently that mRNA vaccines cause autoimmune disease or make it worse, says Betty Diamond, an immunologist and rheumatologist at the Feinstein Institutes for Medical Research of Northwell Health. “At the moment there’s every reason to suggest that people with autoimmune diseases ought to get either of these vaccines when they get rolled out.”
As for more general public acceptance of the vaccines, Weissman notes that the new shingles vaccine, Shingrix, can also cause significant transient reactions. In a large, pivotal trial of people 50 and older, Shingrix caused severe reactions, including pain at the injection site and muscle aches, in 17% of vaccine recipients. Yet demand for that vaccine, licensed in 2017, has been huge.
Hausman says the reported 95% efficacy of both mRNA COVID-19 vaccines bodes well for acceptance. “If you know that a vaccine is really effective, like measles, in making sure you don’t get the infection, then you might be willing to accept a more severe initial reaction.”
Hutchison agrees. Although he hopes better vaccines are on the way, “given that COVID can kill or incapacitate people, everybody should bite the bullet and expect a rough night,” he says. “Get lots of naproxen.”
COVID-19 Update: The connection between local and global issues–the Pulitzer Center's long standing mantra–has, sadly, never been more evident. We are uniquely positioned to serve the journalists, news media organizations, schools, and universities we partner with by continuing to advance our core mission: enabling great journalism and education about underreported and systemic issues that resonate now–and continue to have relevance in times ahead. We believe that this is a moment for decisive action. Learn more about the steps we are taking.