Episode 9: The Fumes in South Portland. The ninth in an ongoing first-person series by InsideClimate News reporter Sabrina Shankman about the growing fears of residents in South Portland, Maine, as they try to solve a mystery: Are the fumes emanating from the storage tanks of the nation's easternmost oil port harming their kids? The series is funded by the Pulitzer Center on Crisis Reporting.
SOUTH PORTLAND, Maine—Before the world turned on its head and we found ourselves locked down at home, navigating a global health crisis, David Falatko was putting in a lot of late nights at his office, trying to take on a different kind of crisis.
Each day, when he finished his work as an environmental engineer, he would switch gears and focus on the environmental threat in his own backyard. Falatko, his wife and their four kids are among thousands of residents of South Portland who live next to massive petroleum storage tanks that are causing concern because they emit chemicals that can cause cancer and other health problems.
Along with others in South Portland, I've been fixated on the tanks for the last year, trying to figure out whether they are emitting enough of these chemicals—called volatile organic compounds, or VOCs—to pose a threat to our health.
But Falatko's professional background has armed him with a skill set that allows him to see things a bit differently, and that led him recently to an "ah-ha" moment, as he dug through documents related to the tanks.
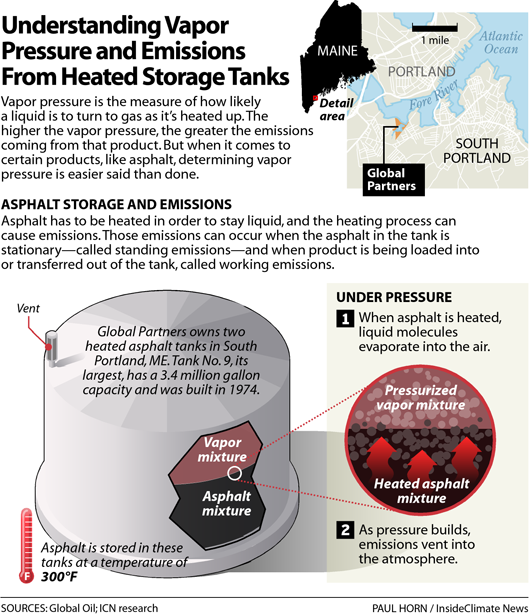
It's been a year since the federal Environmental Protection Agency filed a consent decree against Global Partners, a Massachusetts-based company that owns some of the 120 tanks in South Portland, including four heated tanks that contain asphalt and No. 6 fuel oil, also known as bunker fuel.
According to that decree, Global's tanks had the potential to emit roughly twice the amount of VOCs as the company's permit allowed, in violation of the federal Clean Air Act.
The EPA had been looking into Global's heated tanks since 2011, devising tests to figure out what was being emitted and how much. But no one in the city was aware of that investigation until the consent decree of March 2019.
In a short period of time after finding out about the consent decree, Falatko and the rest of South Portland learned three alarming facts:
- The tanks' emissions are never directly tested but rather calculated using a mathematical formula written by the oil industry.
- Companies self-report their emissions to the state.
- The air quality in the community was not being tested, despite the relative (for Maine) density of the population and the complaints about petroleum odor so severe it could sting the eyes and cause headaches.
When I learned about the consent decree my thoughts—like those of many other people—went to my kids, whose daycare center was a stone's throw from the tanks, and who were sometimes surrounded by the stench—even in my backyard, about a mile from where the tanks are located. I wanted to know: Is it safe for my kids to be breathing this air? A year later, I still don't know the answer, but I'm trying to find out.
The more I learn, the more I find myself in a technical thicket. I didn't study science as an undergraduate. I studied journalism, which basically adds up to being an English major with fewer job prospects.
But I've been pulling this thread about the storage tanks and it's brought me to this: The answer to our questions about what these tanks are emitting—and how much—lies partly in knowing what the vapor pressure is for the products they contain, and the reliability of the tests used to determine it.
But I'll get to that in a moment.
A lot of folks in South Portland are riled up. Like me, they want to know that the air they're breathing is safe, and they want the regulators to impose requirements on Global and the other companies operating here to ensure they can't emit dangerous levels of these chemicals. Though the state is now a year into an air monitoring effort, residents here also want to see that effort stepped up: Specifically, they want air quality monitors closer to Global's tanks, and 24/7 fenceline monitoring established around the perimeter of the facility.
Falatko made his discovery in early March, when he found a letter that Global had sent to the state Department of Environmental Protection—a technical memo most people wouldn't be aware even existed, but that he had learned about through bird-dogging the situation.
By the time he was finished reading the memo, he had realized how precarious this whole process was. There were multiple, federally-approved approaches to calculating emissions. And depending on which method you used to calculate Global's emissions, they could register as higher than previously thought or reported.
Much, much higher.
Different Numbers, Different Results
The memo Falatko found explained that Global had recently changed the way it calculated the emissions coming from its heated tanks. As a result, the tanks' overall emissions appeared to drop—precipitously.
From 2017 to 2018, Global's reported emissions dropped 74.3 percent, from 18.7 tons—not far from the state-issued permit limit of 21.9 tons of VOCs per year—to 4.81 tons. This was despite the fact that there was no comparable change in the amount of product the company moved through its tanks during that time period.
How did this happen?
Since I first started reporting on the storage tanks in South Portland, I've been struck by how fuzzy the math is when it comes to calculating emissions. Regulators give companies some leeway in determining the best way to add up their emissions. But how do you define "best"? Does it mean "most accurate"? Or does it mean "least"?
For about five years leading up to 2018, Global calculated its emissions based on some of the data from the EPA's investigation. That generally resulted in between 17 and 19 tons of reported VOC emissions per year.
But for its 2018 emissions, Global switched it up. They started using a method for estimating emissions that was developed by the oil industry and recommended by the EPA and the state. Called AP42, it was a way to come up with an estimate for the amount of chemicals being emitted from the tanks without actually measuring them. And just like that, their emissions plummeted.
Had they continued to use the approach based on data from the EPA investigation, Global wrote in a footnote to the filing, the 2018 emissions would have been 17.3 tons, instead of the much lower emissions they recorded.
This was part of Falatko's discovery. But it was small potatoes compared to what he found next.
When Global calculated its 2018 emissions using AP42, it plugged an estimated value for the vapor pressure of asphalt into the equation. That matters because the higher the vapor pressure, the greater the emissions. And this, it turned out, was the key to Global's decreased emissions.
The estimated value exists so that companies that don't know the true vapor pressure of their products can still calculate emissions.
But here's the thing: Global already had an idea of what the vapor pressure of its asphalt was, thanks to measurements the EPA required during its investigation.
Back in 2012, at the request of the EPA, Global took a sample from one of its asphalt tanks and sent it off to a lab to have its vapor pressure tested. And that number was much higher than the estimated value contained in AP42.
As Falatko was reading through Global's memo, he remembered seeing documents from the EPA investigation that included those higher results. He pulled them out and got to work.
He started by crunching numbers, reverse engineering the model so he could first understand how Global had calculated its 2018 emissions using AP42, using the oil industry's estimated vapor pressure value. Then he replaced that estimate with the results of the vapor pressure tests from the EPA investigation. Then he checked his math. And checked it again. He ran it by a colleague with a background in chemistry. Then he checked it once more.
The result: By his calculations, the 2018 emissions from just one of Global's asphalt tanks should have been 17.64 tons per year, not the 0.31 tons the company reported. Extrapolating from that increase to the emissions from both of Global's asphalt tanks would result in 35.5 tons per year—a figure that did not even take into account other storage tanks that held non-asphalt products.
There was other evidence to support Falatko's calculation. During the EPA's investigation of Global's heated tanks, it had directly measured the emissions coming from the tanks. Its conclusion: The potential emissions from Global's No. 6 oil and asphalt tanks "alone exceed 40 tons per year," according to the complaint filed by the EPA.
"It really felt like: 'I think I have them now—I have some good hard evidence,'" Falatko said.
But would it be enough to get the state to impose stricter regulations?
When it Comes to Emissions, Nothing is Simple
Falatko explained all this to me in his office in South Portland, one of just a few furnished spaces inside a mostly empty building in a fairly industrial part of town. This was just a few days before we would learn how the coronavirus was about to shrink our worlds.
A couple of days earlier, he had sent his discovery to the state DEP and then forwarded the email to me. I read it and wondered if this was some sort of smoking gun. Later, I would realize how naive that thought was— if I've learned one thing in the last year of reporting on this, it's that nothing is simple when it comes to emissions.
Falatko's discovery was especially relevant because Global is in the process of applying to amend its emissions permit with the state—a requirement of the consent decree with the EPA that would add restrictions on how the company can operate. Some local advocates have seized on that application process as an opportunity to get stronger regulatory oversight of Global's operations.
Right now, Global is considered a "minor source emitter," a designation for facilities that emit less than 50 tons of VOCs a year. If Global were to emit more than that, under federal law the facility would have to be licensed as a major source of emissions, which would subject it to stricter regulations and require the use of the best available control technology to remove as many pollutants as possible.
So Falatko, along with the local grassroots group Protect South Portland, is hoping that this will be their moment, and that they can convince the Maine DEP that Global is a major emitter, rather than a minor one.
Armed with Falatko's discovery, they feel they have proof that Global is violating its emissions permit and perhaps even emitting more than the 50-ton limit that would require regulation as a major source.
After meeting with Falatko, two things become clear. First, I wish I had taken more chemistry classes. And second, I needed to talk to Global to find out why they chose to use the vapor pressure figure they did.
Disagreement Over a Test
Sanjay Srinivasan, the head of the energy and mineral engineering department at Pennsylvania State University, helped me out with the first part.
When you change the temperature of a fluid, he explained—when you heat it up, or cool it down—some of the lightest parts of that fluid can turn into vapor. If that fluid is in a closed container, like a big storage tank, the pressure is going to build up more, because the vapor will accumulate at the top. "So the vapor pressure is very much a function of temperature," he told me.
Temperature is key with asphalt, because it has to be heated to stay liquid. Global heats its asphalt tanks to 300°F.
And asphalt is an especially tricky product, Srinivasan explained, because "every asphalt is not the same. You can put different additives in."
Those additives, which can include a variety of chemicals and even ground tire rubber, improve the asphalt's performance, ensuring, for example, that pavement stays firm when it's hot out or that road surfaces don't wear out prematurely. The additives also have an impact on the vapor pressure.
Which brings us to another reason it matters whether you use an estimated value or test for the actual measurements: Vapor pressure measured by a test would include any additives. But, because different companies use different additives, an estimated value that's not based on measurements might not.
When I hung up with Srinivasan, I was left wondering if maybe those differences from one batch of asphalt to another might explain why Global didn't want to use the results from the 2012 sample. Maybe they felt those results weren't representative of other batches of asphalt that they process?
I reached out to Liz Fuller, a spokeswoman for the company, with a handful of questions, and eventually learned that: No, that wasn't the reason at all.
The test that was used to determine Global's vapor pressure "has issues," she told me via email. "We do not think it is appropriate for asphalt, because the viscosity of the product causes a lot of issues with the test."
Basically she was saying that the test results are hard to replicate consistently when used for asphalt. Because of that, the best guidance from state and federal regulators, Fuller said, is to use AP42, the method developed by the oil industry that includes an estimated value for vapor pressure, as Global did for its 2018 emissions.
But, as I would soon learn, not everyone agrees with her.
Lessons from Pasta
These last few months I've been climbing a steep learning curve, both when it comes to vapor pressure, and surviving a pandemic with two kids under five-years-old.
At home, I've been trying to simplify. Less elaborate Pinterest parenting, more Playdough. Less time searching for secret hikes where we can socially distance, more neighborhood bike rides.
And at mealtime, when in doubt, I default to the one thing my two picky eaters are guaranteed to eat: pasta.
I would never have guessed that this last survival strategy—cooking pasta—would prove helpful in understanding vapor pressure. But here's what I learned.
When you're cooking pasta, long before the water boils, a few rogue bubbles pop up. That's the key to the controversy over the vapor pressure test that was done on Global's sample in the EPA investigation.
The test that was used relies on an important step, called degassing, that removes atmospheric gasses from the asphalt before determining its vapor pressure. It's like those early bubbles in the pasta water, which are likely to be dissolved nitrogen and oxygen rising to the top long before the water boils.
If the degassing isn't conducted accurately in the vapor pressure test, the reading can be off—significantly so. A few years ago, concerns about the test's accuracy led to an industry effort to discredit it and remove it as an option for determining vapor pressure. That effort failed, but the work conducted during that study formed the basis for Global's conclusion that the test was no good, according to Fuller.
As I've talked to experts both on and off-the-record about the test, they've indicated that despite its flaws, if it's done correctly, it may still be the best option. And, they note, it's even recommended in AP42 itself, if done correctly.
But when I show them the vapor pressure test results from Global's sample—the ones that surprised Falatko and differed sharply from the lower, estimated value in AP42—they pause. Those numbers look high, they tell me. Suspiciously so.
Srinivasan was one of two experts I spoke with who wondered if there could have been an error in the calculation of Global's vapor pressure when the company's sample was tested, though he also said he wouldn't dismiss the finding outright. "The range of uncertainty on vapor pressure is so large," he tells me. "That's the reason I cannot balk at this."
I followed up with Fuller, from Global, to see if they wanted to look into the question of whether there was an error in the lab's analysis of the sample before I published my story, but she said the company's staffers were tied up with the coronavirus, and that I should use the comment she had sent me previously.
As part of our initial correspondence, I had sent her Falatko's findings to hear her thoughts, and she got back to me with this: "We appreciate that Mr. Falatko has put a lot of time into this. Air emissions are very technical, and require a background in that body of work. We suggest you consult with regulators if you have questions about the standards."
So that's what I did.
An Argument for Measurement Over Estimate
As a journalist, I've discovered that sometimes the most unexpected information comes from a random Google search. That's how, by a happy accident of search terms, I landed on a page with the pdf of a 2015 presentation from the Texas Commission on Environmental Quality (TCEQ) called "Emissions Inventory: Rule Applicability and What's New?"
Deep into this 244-page presentation, Russ Nettles, a technical specialist in the TCEQ's Air Quality Division, suggested that for heated tanks and hot product storage tanks, like the asphalt or No. 6 fuel oil tanks that Global owns, "do not use AP-42 defaults or other default values for vapor pressure ... unless representative of the stored liquid." Instead, he wrote that the test that was used on Global's sample is "suitable for determining vapor pressure for heavy liquids at the actual storage temperature."
The presentation also said that if a product in a tank is cut with another material—as Srinivasan explained is common practice with products like asphalt—that can change the vapor pressure, and must be taken into account.
It had been a few years since that presentation was written, so I followed up with the TCEQ.
Brian McGovern from TCEQ media relations told me this:
A test based on actual emissions—if done correctly—is more accurate than using the estimated vapor pressure values in AP42. And if they're available, true vapor pressure values should be used.
That included results found using the test that was conducted on Global's sample during the EPA's investigation.
A Public Meeting Postponed
The work that Falatko did in early March was in preparation for what was going to be a big public meeting between the state and South Portland residents about Global's application to amend its permit.
That meeting was supposed to take place on March 31, but by then, the coronavirus was raging across the country and everyone had locked down. The state opted to push back the public comment period on Global's application to June 17, and a meeting is planned on Monday with South Portland's Clean Air Advisory Committee, a group of experts from different fields that has been investigating the emissions issue in the city.
Falatko sent his findings off to the state DEP back in March, but it's still unclear what they will do with them.
I've been asking the DEP about that. Jeff Crawford, the director of the DEP's Bureau of Air Quality, told me they will consider Falatko's comments as they weigh Global's application to amend its license.
He also said that DEP agrees with the Texas regulators that "valid site-specific data is most appropriate and should be used to determine emissions, and that site-specific vapor pressures can be determined using ASTM D2879, as appropriate," Crawford said, referring to the test used on Global's sample.
But that came with some big caveats.
"There is significant difficulty in obtaining valid vapor pressure results for #6 fuel oil and asphalt, the two products stored in Global's heated tanks," he warned.
Because of the concerns about properly degassing the products for the test, and the specific challenges in testing heavy, impure products like asphalt and No. 6 fuel oil, he said, it was questionable whether the test is appropriate for these materials. "The reason that calculated and/or assumed vapor pressure numbers are used so often, is due to the high level of difficulty in obtaining meaningful and repeatable test results," Crawford wrote in an email.
He pointed me toward research out of the University of Texas, Austin that echoed that thought. In painstaking detail, the researchers examined all the possible ways to test the vapor pressure of asphalt and No. 6 fuel oil and concluded that they're all prone to error.
There is no easy answer. But one thing is clear: The estimated vapor pressure values in the industry-developed emissions estimation method are alarmingly low.
"These default values have resulted in estimates of storage tank emissions that are too low by more than an order of magnitude, in some cases several orders of magnitude," the authors wrote in 2015.
But What About the Fumes?
So much of this problem feels so abstract, involving complex chemistry, obscure tests and numbers that are hard for lay-people to understand. But when I wake up at 2 a.m. to calm a crying baby, my bedroom windows wide open to enjoy the cool spring air, here's what it adds up to: That air smells like petroleum.
In the last few months, thanks to the coronavirus, everything about our lives has changed, but the smell remains. Each spring, after a few months of dormancy, Global and other local tank companies heat up their asphalt tanks for the paving season. And in March, the smell came roaring back into our lives.
Some days it doesn't smell. But most days it does. When my husband and I put our kids in the jogging stroller and head out for a run on South Portland's Greenbelt, our eyes meet as we suck in the sharp air near the tanks.
We run past kids playing basketball in their driveway, and mask-wearing adults chatting at a social distance as they walk their dogs. The smell is unmistakable, and I wonder about whether I should be running here with my kids before realizing that many other kids live here all the time.
Now, at a time when we're all staying home, I think about these kids, who no longer have schools to escape to during the day because of a "Safer at Home" emergency order that aims to do exactly that—keep them safer by keeping them home. When it comes to the coronavirus, that makes a lot of sense. But what about the fumes?